| The
Andhra Journal of Industrial News Chief Editor: Prof. Sreenivasarao Vepachedu (Click here to subscribe to this free e-journal) |
|
|
| |
To join The Indian American Chemical
Society (TIACS), please send an email to: TIACS-subscribe@yahoogroups.com |
||
|
|
|
||
| Home Management AJIN TSJ MS Vegetarian Links Disclaimer Soliciataion Contact VPC More Links Vedah |
Contents
Patent Law in the Philippines GENERIC V. INNOVATOR Abbott Acquires Solvay Search for New Drugs US is the New Third World UK- Life Sciences Super Cluster Mergers and Acquisitions in 2009 Sanofi Cuts Research Proper Assignments Recalculation Of U.S. Patent Terms Patenting Strategy 50% of counterfeit goods come from China Pakistan’s a Threat Indian Medical Devices Technology Park Patent Law in the Philippines The Philippines Quality Medicine Act amends the IP Code to state that the mere discovery of a new form of a new property of a known substance which does not result in the enhancement of the known efficacy of that substance, or the mere discovery of any new property or new use for a known substance, or the mere use of a known process unless such known process results in a new product that employs at least one new reactant is non-patentable. In addition, a patent owner has no right to prevent third parties from using a patent without his authorization after a drug or medicine has been introduced in the Philippines or anywhere else in the world by the patent holder or by any party authorized to use the invention. The act also grants the right to import drugs and medicines to any government agency or any private third party. In the case of drugs and medicines, the patent owner has no right to prevent the testing, using, making or selling of the invention including any data related thereto, solely for purposes reasonably related to the development and submission of information and issuance of approvals by government regulatory organizations required under any law of the Philippines, or of another country that regulates the manufacture, construction, use or sale of any product. Upon recommendation of the secretary of the Department of Health, the director-general of the Intellectual Property Office can grant a compulsory license for the importation of patented drugs and medicines, with reasonable compensation to the patent owner. The president of the Philippines also has the power to impose maximum retail prices over any or all drugs and medicines listed in Section 23 of the act, which include: those indicated for treatment of chronic illnesses and life threatening conditions such as, but not limited to, endocrine disorders, cardiovascular diseases, skin diseases, pulmonary diseases, neuropsychiatric disorders or other infectious diseases; those indicated for prevention of pregnancy; and anesthetic agents. The Philippine Patent No. 29149 for Atorvastatin, marketed under the brand name Lipitor, was issued by the IPO after having gone through a thorough examination and was deemed to meet all the criteria for patentability based on the above Philippine laws. Pfizer filed a complaint for patent infringement and damages against Unilab for selling its generic anticholesterol drug Avamax, which is priced 30 percent lower than Pfizer’s. Unilab claims that according to the IP Code, as amended by the Cheaper Medicines Act, the patent should not have been granted since it failed to meet the requirements for patentability. Unilab claims the patent is merely an attempt at “evergreening,” an abuse of the patent system by patent holders that seek to perpetuate their monopoly over a product beyond the period allowed by law. Unilab contends that there is nothing inventive about the Atorvastatin covered by Patent No. 29149 since it is nothing but an isomer of the Atrovastatin compound already disclosed in U.S. Patent No. 4,681,893 (which discloses NCE atorvastatin) and Philippine Patent Nos. 24661 and 26330. So, the question is why does Unilab not make NCE which is off patent, instead of selling the isomer which is covered by the patent? Obviously, the isomer is better than the NCE. Of course, according to reports, Lipitor (the isomer) was the largest-selling drug in the world in 2006, earning its producers $12.9 billion in sales the same year alone. In 2008, Lipitor sales internationally were at $13 billion. Now you know why Unilab wants to make the isomer and seeks to invalidate the patent, not anything else. Not because poor people of the Philippines are suffering from overeating rich fatty foods, obesity or cholesterol problems! It is all about money and who makes it. WHY ARE DRUGS EXPENSIVE IN THE U.S.? WHILE AMERICA PAYS FOR RESEARCH AND DEVELOPMENT OF NEW DRUGS, OTHER COUNTRIES TAKE PROFITS AND CREDIT IN THE NAME OF HELPING POOR. GENERIC V. INNOVATOR Shortly after he became chief executive officer of Eli Lilly & Co. in April 2008, John Lechleiter, a former lab scientist, sent his senior executives a gift. It was a small digital clock counting down, second-by- second, to Oct. 23, 2011. That’s the day the drugmaker’s $5- billion-a-year schizophrenia pill, Zyprexa, goes off patent. Next to the countdown were four words: “Do what we do.” Lilly must pick up the pace of drug development so it can replace revenue lost when three top- selling medicines lose patent protection in the next few years, Lechleiter said in an interview in his Indianapolis office. The company stands to lose $10 billion in annual sales to generic competition by the end of 2016, almost half of its 2009 revenue, one of the steepest percentage losses resulting from patent expirations among the six biggest U.S. drugmakers. When Lilly faced challenges before, “each and every time the answer has been new, innovative products,” said Lechleiter, whose office wall displays the Periodic Table of Elements, the foundation tool for chemists, his first career as a Ph.D. from Harvard University in Cambridge, Massachusetts. Lilly’s blood-thinner Effient, approved in July, only pulled in $27 million in revenue in 2009, competing against Bristol-Myers Squibb Co.’s $6 billion-a-year Plavix. Lilly suffered two high-profile failures in July and August, when a multiple sclerosis drug candidate and an osteoporosis product fell short of expectations in clinical trials; while generic companies keep looking for the next blockbuster to poach to make money in the name of poor people. While the governments of so-called poor countries do nothing to support innovation of new drugs in their countries, the American government spends millions of dollars in support of new drugs, in addition to billions of dollars spent by the innovator industry. Pharmaceutical companies screen thousands of compounds for the ability to bind a target before they hit upon a promising drug candidate, which will further go through a rigorous process of evaluation involving billions of dollars, e.g., U.S. drug safety reviewers have recommended that GlaxoSmithKline PLC's diabetes drug Avandia be pulled from the market after concluding it is more dangerous to the heart than a rival medicine. Recently, the U.S. Food and Drug Administration and the National Institutes of Health unveiled an initiative designed to accelerate the process from scientific breakthrough to the availability of new, innovative medical therapies for patients. The initiative involves two interrelated scientific disciplines: translational science, the shaping of basic scientific discoveries into treatments; and regulatory science, the development and use of new tools, standards and approaches, to more efficiently develop products and to more effectively evaluate product safety, efficacy and quality. Both disciplines are needed to turn biomedical discoveries into products that benefit people. In addition, the NIH and the FDA will jointly issue a Request for Applications, making $6.75 million available over three years for work in regulatory science. The research supported through this initiative should add to the scientific knowledge base by providing new methods, models or technologies that will inform the scientific and regulatory community about better approaches to evaluating safety and efficacy in medical product development. The effort will rely on the NIH's vast experience supporting and facilitating new discoveries in the laboratory and clinic and the FDA's more than 100 years of experience and knowledge in the regulation and approval of drugs, biologics and medical devices. Abbott Acquires Solvay Abbott announced Feb. 16 that it has completed its $6.2 billion acquisition of Belgium-based Solvay Pharmaceuticals, “providing Abbott with a large and complementary portfolio of pharmaceutical products and expanding Abbott’s presence in key global emerging markets.” The acquisition also includes Solvay’s vaccines business, which will provide Abbott entry into the expanding global vaccines market. Solvay also has a small molecular diagnostics unit that will become part of Abbott’s diagnostics organization upon the transaction close. Abbott notes that it has a strong portfolio of specialty pharmaceuticals and Solvay brings successful, consistently performing products—including branded generics—that will further diversify Abbott’s pharmaceutical business. These products complement Abbott’s presence and expertise in specialty markets such as cardiovascular disease, neuroscience and gastroenterology, and include treatments for men’s and women’s hormonal health, and exocrine pancreatic insufficiency, which is associated with several underlying conditions such as cystic fibrosis and chronic pancreatitis. Solvay Pharmaceuticals is now part of Abbott’s global Pharmaceutical Products Group. Werner Cautreels, CEO of Solvay Pharmaceuticals, will serve in a transitional role and will then leave the company. Based on the timing of the close, Abbott expects the acquisition to add approximately $2.9 billion to Abbott’s 2010 total reported sales—only slightly down from the September prediction of $3 billion—with the majority of those sales outside the U.S. The acquisition will also add approximately $500 million to Abbott’s annual pharmaceutical research and development investments. Search for New Drugs The search for new therapeutic agents is time-consuming and expensive. Pharmaceutical companies may have to screen thousands of compounds for the ability to bind a target molecule before they hit upon a promising drug candidate. A group of Biophysicists at LMU Munich led by Professor Dieter Braun, a member of the Cluster of Excellence "Nanosystems Initiative Munich" (NIM), and a partner in NanoTemper (an LMU spin-off), have now developed a unique technology called "microscale thermophoresis" that allows to measure intereactions under close-to-native conditions, thus improving the decision making process in drug development. The technique takes advantage of the Soret effect – the tendency of molecules to drift along temperature gradients, usually from warm to cold. If a compound encounters and binds to another molecule, its thermophoretic parameters change, and its trajectory may even be reversed. This phenomenon can be exploited to determine whether a molecule that is known to play a causative role in a given disease binds to a test substance. In the test, which can be carried out directly on blood samples, the thermodiffusion of a labelled biomolecule of interest is measured in the presence and absence of a candidate binding agent. If the two bind together to form a complex, the resulting change in their thermophoretic behaviour can be detected. "Detection of binding activity is the first step on the road to a new drug", says Braun. "The new method also has potential applications in medical diagnostics, and in food and environmental monitoring." http://www.eurekalert.org/pub_releases/2010-02/lm-ahr022410.php US is the New Third World Cook county state's attorney anita alvarez told a u.s. senate hearing that some young chicagoans are practicing "survival sex" and selling their bodies for food, clothing or a safe place to sleep, Alvarez, addressing a subcommittee looking into human trafficking, told of a girl who didn't want her pimp to face charges because he bought her a subway sandwich whenever she wanted one. another girl had sex for cash to buy food and clothing, unable to rely on her mother, a drug addict. The hearing was called by Sen. Dick Durbin, D-Ill., chairman of the Subcommittee on Human Rights and the Law. He estimated that 100,000-plus U.S. children become sex-trafficking victims every year. Compare this to India with 1.3 billion ppulation. The Ministry of Women and Child Development reported presence of 2.8 million sex workers in India, with 35.47 percent of them entering the trade before the age of 18 years. (http://en.wikipedia.org/wiki/Prostitution_in_India) UK- Life Sciences Super Cluster The U.K. Life Sciences industry employs over 120,000 people and invests approximately £4.6 billion into R&D. The U.K. government has recently announced their plans for a new U.K. Life Sciences Super Cluster - a scheme where industry, academia and the National Health Service work together to deliver new treatments for chronic disease. The pilot project is being supported by a £1 Million investment from the government and the first Super Cluster will launch later this year in the area of immunology and inflammation, focusing on diseases such as asthma and rheumatoid arthritis. The unveiling of this new scheme comes in addition to recent measures to help the U.K. maintain a competitive edge in translational medicine, including the Patent box and Innovation Pass initiatives, to better compete on a global stage and attract inward investment. http://nds.coi.gov.uk/clientmicrosite/content/Detail.aspx?ReleaseID=410611&NewsAreaID=2&ClientID=431 Generics in Europe The European Generic Medicines Association (EGA) is advocating for changes to regulatory framework of the European Union with respect to the marketing of generics and biosimilars in the European Union. The EGA is seeking to enhance tax and R&D incentives for generic and biosimilar research and clinical trials. The generic manufacturers are also seeking to expedite market authorizations by eliminating patent linkage and applying a harmonized single market approach to approvals. http://www.egagenerics.com/pr-2010-01-21.htm The European Commission continues to scrutinize the pharmaceutical sector and patent settlement agreements between innovator and generic pharmaceutical companies. The European Commission's July 2009 Pharmaceutical Sector Inquiry Report focused on the behavior and practices of the "originator industry." It was alleged that innovators had caused undue delays in generic market entry. The Report appears to have prompted legal proceedings against pharmaceutical companies such as Servier and Lundbeck and the European Commission has recently sent "requests for information" to a number of other pharmaceutical companies seeking copies of patent settlement agreements conducted between innovator and generic companies executed in the last 18 months. Further, a number of unannounced inspections targeting the legal departments of many large pharmaceutical companies of were conducted in late 2009. The Commission's Report notes concerns relating to 'defensive' patents and regulatory complaint strategies. http://www.pharmatimes.com/WorldNews/article.aspx?id=17288 Mergers and Acquisitions in 2009 In 2009, the landscape of mergers and acquisitions (M&As) by the top 50 pharmaceutical companies (ranked by sales in 2008) was dominated by mega-mergers, with three deals exceeding the US$40 billion mark. Beyond these deals there was significant, if smaller, activity, with 21 of these companies embracing M&As as part of a diverse range of strategies. Overall, the 50 top-tier companies were involved in 44 completed M&A deals in 2009, a small increase above the 42 such deals completed in 2008. The total value of the deals in 2009 was a massive $190 billion compared with $48 billion in 2008, but more than 80% of the 2009 figure is attributable to just three deals: Pfizer–Wyeth ($68 billion), Roche–Genentech ($47 billion) and Merck–Schering-Plough ($41 billion). Taking this into account, the remaining spending of $34 billion represents a reduction of around 30% compared with 2008…. Market expansion was also a major driver of acquisitions in 2009, with companies seeking to expand their presence in their current operational markets as well as to enter other new regions, including the emerging markets. The most active company in this area was Sanofi–Aventis, which spent more than $3 billion on three deals to expand its presence in India, Mexico and Brazil. http://www.nature.com/nrd/journal/v9/n2/pdf/nrd3114.pdf Sanofi Cuts Research Sanofi-Aventis has been cutting down the amount of money it's spending on R&D. In its annual report, the pharma giant revealed that it had shaved seven percent out of its R&D budget last year as it restructured its pipeline. After recounting its success advancing new drugs for ovarian cancer and mid-stage vaccine programs, Sanofi,which has recently inked a series of new collaborations with biotech companies like Regeneron and Syntiron, also said that it had decided to drop its program for the cancer drug larotaxel and had terminated six Phase I development projects. Sanofi’s R&D portfolio is comprised of 49 projects in clinical development of which 17 are in Phase III or have been submitted to the health authorities for approval. Proper Assignments Board of Trustees of the Leland Stanford Junior University v. Roche Molecular Systems, Inc., 583 F.3d 832 (Fed. Cir. 2009), highlights the critical importance of proper assignment language in contracts which purport to transfer an inventor's rights in his or her inventions to the entity with whom the inventor is employed or otherwise engaged. A contract which merely provides that the inventor "agrees to assign" his rights vests the assignee with, at most, equitable title in the inventions. A subsequent contract in which the inventor "hereby assigns" those same rights to a separate assignee will defeat the earlier assignment. http://www.mondaq.com/unitedstates/article.asp?article_id=93890&lk=2 Recalculation Of U.S. Patent Terms The United States Patent and Trademark Office (PTO) has released guidelines explaining how patentees of recently issued patents may request the recalculation of the terms of their patents. This guidance arose from the Court of Appeals decision in Wyeth v. Kappos in January 2010, which held that the PTO had previously misapplied the statutory provisions relating to patent term adjustments. … Patentees considering seeking recalculation of their patent terms should also assess whether to concurrently pursue a civil action, especially if they are submitting their recalculation requests to the PTO close to the end of the 180-day period and if it is unclear whether the PTO will confirm a correction by the expiry of the period for requesting reconsideration. http://www.mondaq.com/unitedstates/article.asp?articleid=93428&email_access=on The USPTO has not yet indicated whether it will apply Wyeth retroactively. Under 37 C.F.R. § 1.705(d), a request for reconsideration of patent term adjustment must be filed within two months of patent issuance. However, it may be advisable for certain patent owners to request that the USPTO suspend the two-month limitation on requests for reconsideration in order to comply with correct patent term adjustment re-calculation. Petitions for correction of an erroneous term should be filed sooner rather than later to reflect appropriate diligence. Patentees that have any questions about Wyeth or the potential for requesting an additional patent term should contact a patent attorney in the near future. Idera Pharmaceuticals is challenging recently enacted interim procedures for revising patent term extensions as being inequitable. Idera filed suit on January 29, 2010, asserting that the Patent Office calculation ("Patent Office new math") truncated extension days from two of their patents and challenging the equity of the interim procedures. Idera alleges in the complaint (http://react.bracewellgiuliani.com/reaction/announcements/10_166_1.pdf) that the Wyeth decision "constitutes a change in law sufficient to invoke the doctrine of equitable tolling" with regard to the '554 patent. Equitable tolling, as explained by the Federal Circuit in Serdarevic v. Advanced Medical Optics Inc., 532 F.3d 1352, 1363 (Fed. Cir. 2008) on the doctrine, will not allow a statue of limitations to run against a plaintiff who is unaware of its possible cause of action. Patenting Strategy Filing patent applications needs to be carefully planned to obtain maximum benefit from the patent system. Industries based on life science take considerably more time to develop a product than those based on mechanical, electrical and software, because testing of inventions on live subjects takes time and money, while mechanical, electrical and software based inventions can be tested and improved rapidly. Within a year of the filing of a provisional application, a complete international application should be prepared and filed along with applications in any non-PCT countries of interest. Signaling your intentions to competitors, a publication of the patent application occurs six months after this, i.e., 18 months from the priority filing. Then, at the 30/31 month, the applicant has to enter national stage in PCT countries of interest. This timing may not suit the long development cycles in the drug and agricultural industry. To warrant the expensive filing of international patent applications, one needs to know if the product is likely to work, whether there is a market and the final form of the product so that you can get robust patent protection. It is possible to delay filing applications or shift filing dates of applications if a particular project is taking longer than expected to become market ready. However, one has to watch out for research publications that are unwisely tied to promotions and bonuses of scientists by the management in the industry, because publication before patent application filing adversely affects the patentability and the ability to juggle priority dates. It may be a good idea to delay filing a patent application until you have some confidence in the invention. However, in a highly competitive area, the risk of losing the priority date is imminent. Withdrawing an early filed provisional patent application and re-filing it is an option. However, it should be considered only when you are confident that there are no intervening publications or applications filed by your competition. Filing a broad generic patent application for a concept is dangerous because of the risk that without specific examples it may not get granted. However, this can act as a deterrent against competition until you file a more specific embodiment of the invention in a continuation-in-part or a selection application which requires unexpected results over the generic disclosure. It is essential to have a proper IP strategy and to have controls in place to prevent premature publications. China: 50% of counterfeit goods come from China 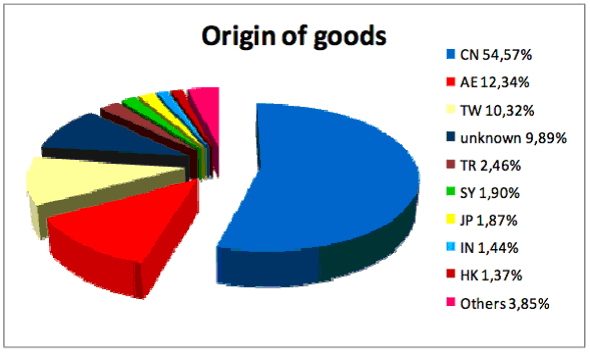
"China was the main source country for [intellectual property rights] infringing articles with 54% of the total amount." http://digg.com/business_finance/15_Facts_About_China_That_Will_Blow_Your_Mind Pakistan’s a Threat Pakistan is rapidly becoming the fourth most populous country in the world and lacks the infrastructure for educating its surging population and the economy for employing them. “Time is running out to put appropriate policies in place. The absence of this may result in large-scale unemployment and immense pressure on health and education systems. In short, a socio-economic crisis may take place, making the demographic dividend more of a demographic threat,” said Durr-e-Nayab of the Pakistan Institute of Development Economics. History shows that economic instability increases the potential for conflict among nations. No wonder Pakistan has become the center of terrorism. Indian Medical Devices Technology Park Medical devices and supplies market in India is expected touch USD 1.7 billion in 2010, growing at the rate of 23% annually in the coming years from the current Rs. 5750 crores, according to a recent sector report by National Institute of Pharmaceutical Education and Research by (NIPER), Ahmedabad. Affordability by patients, increased awareness on health care, improved hospital infrastructure and the increased disease patterns are listed as the primary drivers boosting the growth medical devices industry. Free market environment, a developed industry and investment in health infrastructure are amongst other factors that the growth of high quality medical devices industry. Despite strong growth rates, the market remains disproportionately small, ranking among the top 20 in the world, but with a very low per capita spending. Among the segments in the medical devices market diagnostic equipment leads with Rs. 2000 crores, surgical equipment supplies worth Rs 1300 crores comes second and imaging and electronic treatment devices follow with Rs 1300 crores and Rs. 1000 crores respectively. The segment, including the medical instruments and appliances used in specialties such as ophthalmic, dental and other physiological classes, accounts for 26% of the total market followed by orthopedic/ prosthetic goods segment accounting for 19% of the total market. Medical supplies such as bandages and disposables such as syringes, needles and catheters together constituted 21% of the total market. The other equipments which are in demand are high end specialty electro medical equipments that accounted for 11% of the total market. X-ray apparatus accounted for 10% of the total market. Another high growth segment in the medical devices industry is diagnostic kits. Diagnostic kits have a growth rate of 30% and a market size of USD 133 million in 2005. They include the reagents and the medical kits. With over fifty companies operating in diagnostic kits, the market has seen several interesting trends. Imports constitute over 50% of the market. Most imported products have high gross margins. Currently, the high value imported products include cancer diagnostic, medical imaging, ultrasonic scanning, plastic surgery equipment and polymerase chain reaction technologies. The medical devices market for exports from India is estimated around USD 509 million with a CAGR of 22.15%. It is expected that indigenous production of world-class medical devices will also bring down the overall healthcare costs to considerably low levels. Accordingly, a medical technology park dedicated to the production of medical and pharmaceutical products and equipment has been launched at Irungattukottai near Chennai, the capital city of the southern Indian state of Tamil Nadu. The new facility, named Trivitron Medical Technologies Park, is a joint venture of the leading medical technology company Trivitron Healthcare Private Ltd and Aloka Ltd of Japan, a pioneer in the diagnostic ultrasound technology. The 25 acres-Trivitron Medical Technologies Park is designed to house 10 international medical technology manufacturers. Trivitron Medical Technologies Park’s neraly 20,000 sq ft facility includes internal corridors, raw material storage area, material IN & UT areas have an epoxy coated flooring etc. Medical products and equipment ranging from ultrasound systems, color dopplers, X–ray machines, C-arms , in-vitro diagnostic reagents and instruments, cardiology diagnostic instruments, critical care instruments, modular operating theatres, operating theatre lights and tables and implantable medical devices will be manufactured at Trivitron Medical Technologies Park. Trivitron Healthcare Pvt Ltd has already signed joint ventures with leading international medical devices manufacturers including Brandon Medical, UK, ET, Cardio line, Italy, Johnson Medical, Sweden and Bio-systems, Spain for setting up more facilities at the park. The exports mainly consist of dental instruments, surgical items and other laboratory equipment. Prominent MNC’s operating in the Indian market include B Braun, Becton, Dickinson and company, Bayer, Johnson and Johnson, Phillips, Roche, Siemens and GE. Some of the domestic players hat have consolidated their market position include, BPL Healthcare, Godrej Healthcare, Nicholas Piramal India Ltd., Opto Circuits India Ltd. and Advanced Micronic Devices Ltd. Roche Diagnostics is the leading player followed by Transasia, Bayer and others such as Span Diagnostics, Piramal Healthcare, Orchid, Tulip Diagnostics, Zephyr Biomedical, Biorad, Liliac etc. India is also in the process of finalizing the guidelines to the manufacture, import and sale of medical devices under Schedule M III. The Drugs Technical Advisory Board (DTAB) which provides technical guidance to the Central Drugs Standard Control Organization – the apex body to regulate drugs and cosmetics – under the ministry of Health and Family Welfare has submitted the final draft of the guidelines on medical devices to the drug controller. The medical devices and equipment are currently notified as drugs that are regulated under the Drugs & Cosmetics Act and Rules. However, the guidelines will apply to inspections and other requirements under Schedule M III, which comes as an extension to the Schedule M under India’s Drug and Cosmetics Act that stipulates norms for the manufacturing and sales of pharmaceutical products in the country. In 2010, the medical devices sector in India got a booster dose from the Union Budget 2010-11 that has initiated some measures towards fostering the sector. The Union Finance Ministry has brought in a uniform concessional basic duty of 5% for all medical appliances besides exempting import duty from specified inputs for the manufacture of orthopedic implants, are good initiatives. The budget proposes to prescribe a uniform, concessional basic duty of 5 per cent, countervailing duty (CVD) of 4 per cent with full exemption from special additional duty on all medical equipment. A concessional basic duty of 5 per cent is being prescribed on parts and accessories for the manufacture of such equipment while they would be exempt from CVD and special additional duty. Full exemption currently available to medical equipment and devices such as assistive devices, rehabilitation aids etc. is being retained. The concession available to government hospitals or hospitals set up under a statute is also being retained. For the manufacturers of orthopaedic implants, the Budget 2010-11 has proposed an exemption of specified inputs for the manufacture of implants from import duty. The manufacturers complained that their inputs attracted a higher rate of duty than the finished product. Orthopedic/ prosthetic goods segment currently accounts for 19% of the current Rs. 5750 crores total market for medical devices and equipment. These incentives will definitely spur the growth momentum of the already fast-emerging medical devices and equipment sector in India. The ministry has also announced some tax incentives for the business of setting up and operating “cold chain” infrastructure, which is an integral part in the logistics for vaccines and many biotech products. An Increase in weighted deduction on R&D expenditure up to 200 percent on in-house research and development (R&D) expenses is one of the highlights of Union Budget 2010-11. The budget also proposes to enhance the weighted deduction on payments made to national laboratories, research associations, colleges, universities and other institutions, for scientific research from 125 per cent to 175 per cent. The excise duty on formulations will also remain unchanged at 4 percent. The Budget has also proposed an increase in the plan allocation for the Ministry of Health and Family Welfare from Rs 19,534 crore to Rs 22,300 crore for the financial year 2010-11. http://www.dancewithshadows.com/pillscribe/indian-medical-devices-market-gets-a-booster-dose-from-union-budget-2010-11/ http://www.dancewithshadows.com/pillscribe/india-opens-medical-devices-production-park-in-chennai/ Source: The primary sources cited above, BBC News, New York Times (NYT), Washington Post (WP), Mercury News, Bayarea.com, Chicago Tribune, CNN, USA Today, Intellihealthnews, Deccan Chronicle (DC), the Hindu, Hindustan Times, Times of India, AP, Reuters, AFP, Biospace etc. Notice: The content of the articles is intended to provide general information. Specialist advice should be sought about your specific circumstances. |
||
(Om! Lead the world from wrong path to the right path, from ignorance to knowledge, from mortality to immortality and peace!)
One World One Family
|
|
Management |
The Andhra Journal of
Industrial News |
The Telangana Science
Journal |
Mana Sanskriti (Our
Culture) Journal |
Disclaimer | Solicitation |
Contact |
VPC |

